Vitra 810 for Subcyclo: a new step forward in Subliminal Cyclophotocoagulation
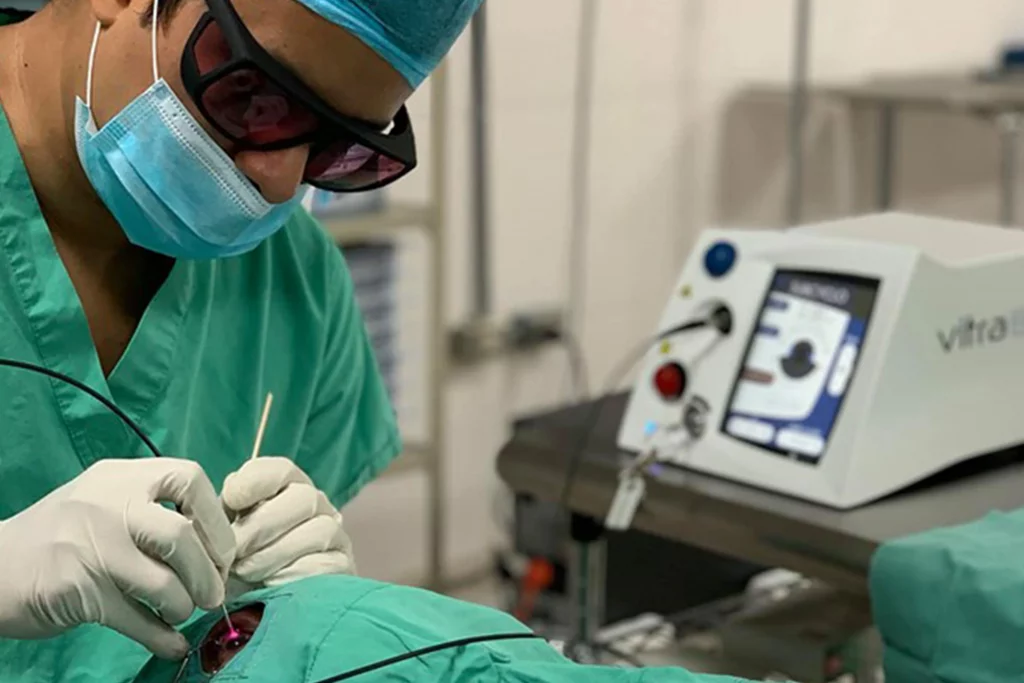
Quantel Medical, a global ophthalmic medical device company dedicated to developing leading technologies to improve the diagnosis and treatment of ocular diseases, today announced the launch of the new VITRA 810TM laser and its new SubCyclo® probe at the American Academy of Ophthalmology (AAO 2018) meeting being held in Chicago, USA, October 27…
ABSolu: new A/B/S/UBM ultrasound platform
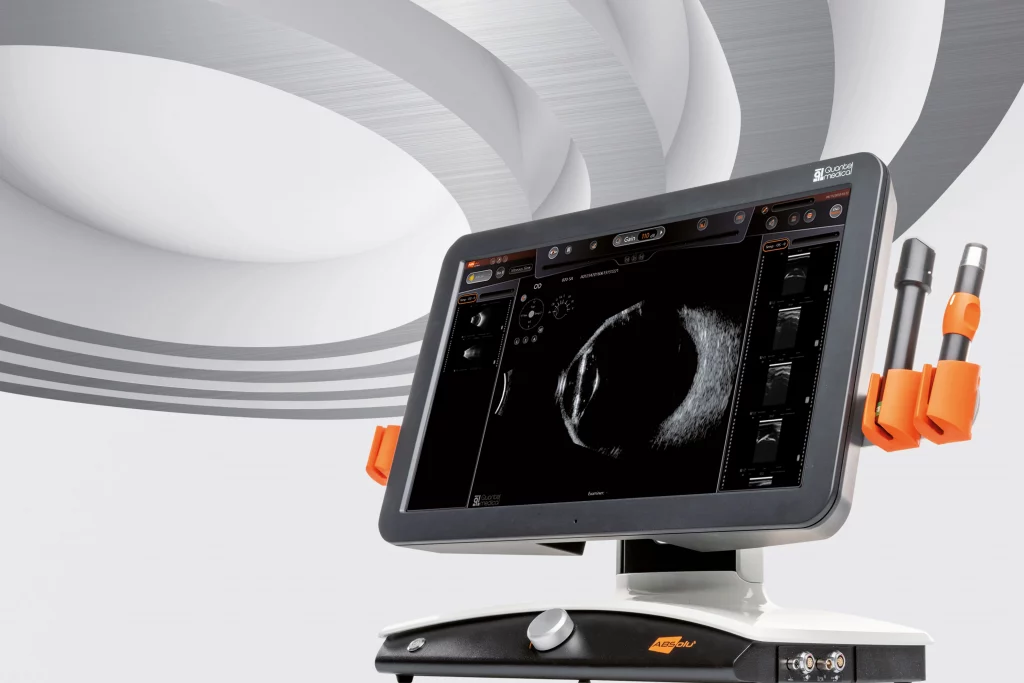
Quantel Medical today announced the launch of the new ABSoluTM ultrasound platform at the 36th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS 2018) in Vienna, Austria September 22-26. Combination of leading edge technologies, this A/B/S/UBM ultrasound platform provides unparalleled image quality with an exceptional…
VITRA 2: Retinal Photocoagulation simplified

Quantel Medical reinvents its flagship 532nm green photocoagulator. Vitra 2 includes all the key elements that have made the success of the Vitra photocoagulator such as reliability, compactness and versatility.
Quantel Medical opens up new markets with its acquisition of ECM’s medical activities

Quantel Medical, a LUMIBIRD Group subsidiary, has taken a further step forward with its development by acquiring the medical activities of ECM, an IMV Technologies group subsidiary. World leader for ophthalmic diagnosis ultrasound solutions, Quantel Medical is opening up to other markets such as sports, general and hospital medicine.
Lacrydiag: complete diagnosis of the dry eye
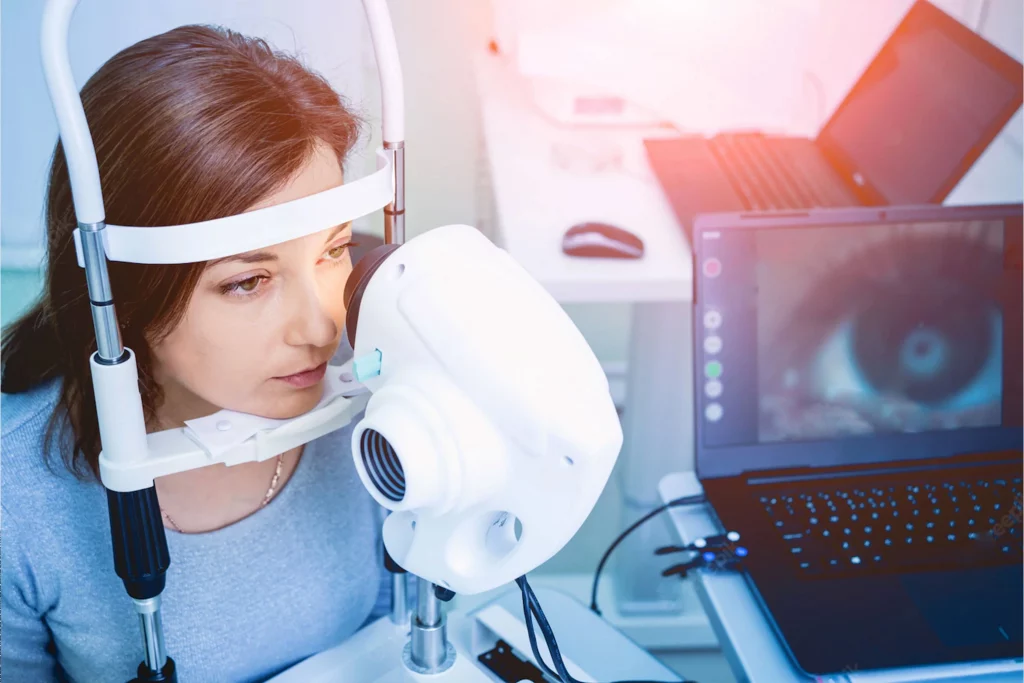
During the last World Ophthalmology Congress held in Barcelona, Spain, June 17-19, Quantel Medical launched LacryDiag, a complete diagnostic device for dry eye. LacryDiag is the first step of Quantel Medical in the dry eye market.
Quantel Medical creates a new subsidiary in Poland

Quantel Medical announces the opening of its new Polish subsidiary: Quantel Medical Polska. This company will commercialize the complete range of ultrasound, laser and dry eye diagnostics products. Quantel Medical Polska has also been appointed exclusive distributor for the products of FCI company (a Zeiss group company).
Quantel Medical receives FDA approval for next-generation Compact Touch ophthalmic ultrasound platform

Quantel Medical today announced that it has received approval from the U.S. Food and Drug Administration (FDA) on March 02, 2018 for the Next-Generation Compact Touch Ophthalmic Ultrasound Platform.
Quantel Medical receives japanese approval for Easyret™ photocoagulator laser

Quantel Medical today announced that it has received the approval for the Japanese market for the innovative new Easyret™ fully integrated 577nm yellow photocoagulator, which is used to treat certain forms of macular edema and peripheral retinal pathologies.
Quantel Medical introduces next-generation Compact Touch ophthalmic ultrasound platform at ESCRS 2017

Quantel Medical (Quantel), a global ophthalmic medical device company dedicated to developing leading technologies to improve the diagnosis and treatment of ocular diseases, today announced the launch of the new generation of the Compact Touch ophthalmic ultrasound platform at the 35th Congress of the European Society of Cataract and Refractive Surgeons…
Quantel Medical receives FDA approval for Easyret photocoagulator laser
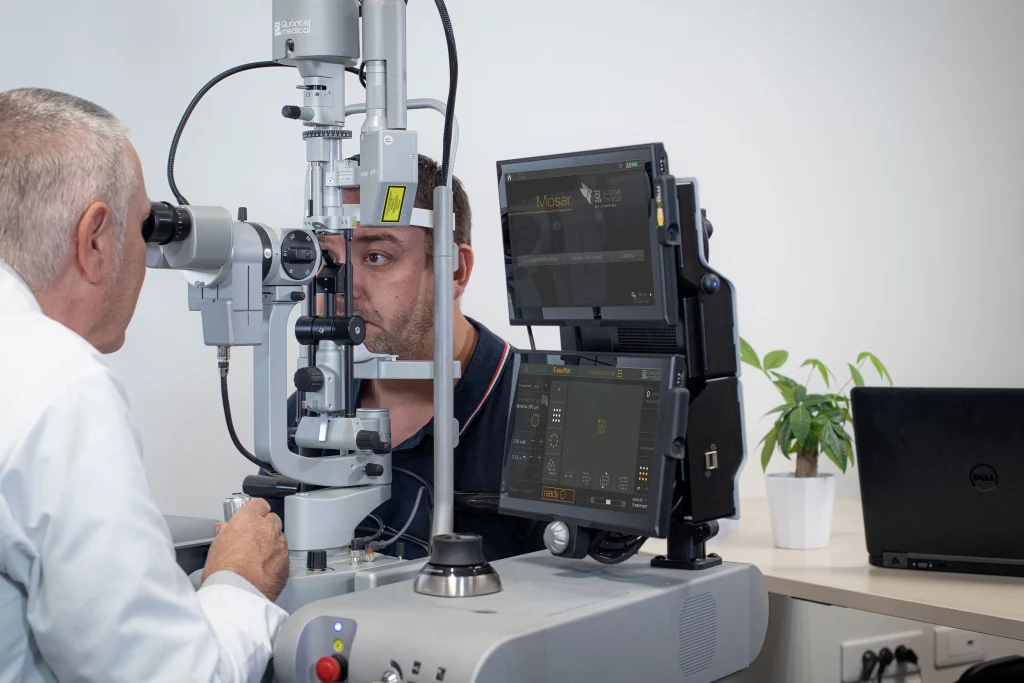
Quantel Medical today announced that it has received approval from the U.S. Food and Drug Administration (FDA) on July 25, 2017 for the innovative new Easyret fully integrated 577nm yellow photocoagulator, which is used to treat certain forms of macular edema and peripheral retinal pathologies.